

Ethers do not have a designated suffix like the other types of molecules we have named so far. In this case, since the –OH is attached to carbon 2 in the chain, we would name this molecule 2-pentanol.Įthers are compounds that contain the functional group –O–. To address the fact that the hydroxyl group is present, we change the ending of the name to -ol. If the hydroxyl group was not present, we would have named this molecule pentane. The carbon chain contains five carbon atoms. Examples include 1,2-ethanediol (ethylene glycol, used in antifreeze) and 1,2,3-propanetriol (glycerine, used as a solvent for cosmetics and medicines):Ĭonsider the following example. Names and position of the substituents.Īlcohols containing two or more hydroxyl groups can be made. And when numbering the parent chain, the hydroxyl group gets the lowest possible number. Identify the longest chain of carbons which contains the OH group and its position (PREFIX-#-AN E+OL). Therefore when naming alcohols following IUPAC, you follow the similar two rules for alkanes with modification to “rule 1” mentioned above.
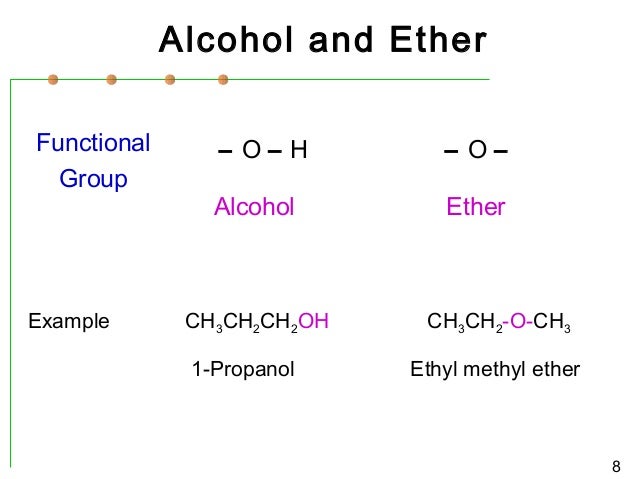
The final -e in the name of the hydrocarbon is replaced by -ol, and the carbon atom to which the hydroxyl group (OH group) is bonded is indicated by a number placed before the name. The name of an alcohol comes from the hydrocarbon from which it was derived. Name and draw structures for alcohols and ethers.Describe the structure and properties of ethers.Describe the structure and properties of alcohols.


 0 kommentar(er)
0 kommentar(er)
